Guest Group: Cilia and Stem Cells in Sensory Systems
PI: Andreas Gießl
Focus
My group has a broad background and expertise in molecular biology and neuroscience. My research includes the analysis of disease mechanisms and fundamental processes in the cornea, photoreceptor cells and ciliogenesis in general. The main interests are the basic cellular mechanisms by which cytoskeletal elements and protein complexes work together to regulate the distribution of molecules in the morphologically and functionally distinct compartments of these tissues.
Possible role of proteins in the ciliary transport in ciliated sensory Neurons
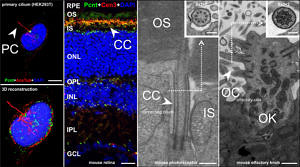 One spotlight in ciliary research is the protein Pericentrin (Pcnt) – also known in human as Kendrin. This protein is implicated in many diseases and disorders, including congenital disorders such as microcephalic osteodysplastic primordial dwarfism type II (MOPDII) and Seckel syndrome. My group studies the mammalian centrosomal/ciliary scaffold protein in the retina and created an interactome of Pcnt through various experiments. In the retina, Pcnt co-localizes with the whole protein transport machinery from the inner to the outer segment of photoreceptor cells. Studying Pcnt function may help us to understand the regulation of protein transport in photoreceptor cells and provide new insights into human disorders related to defects in ciliary function.
One spotlight in ciliary research is the protein Pericentrin (Pcnt) – also known in human as Kendrin. This protein is implicated in many diseases and disorders, including congenital disorders such as microcephalic osteodysplastic primordial dwarfism type II (MOPDII) and Seckel syndrome. My group studies the mammalian centrosomal/ciliary scaffold protein in the retina and created an interactome of Pcnt through various experiments. In the retina, Pcnt co-localizes with the whole protein transport machinery from the inner to the outer segment of photoreceptor cells. Studying Pcnt function may help us to understand the regulation of protein transport in photoreceptor cells and provide new insights into human disorders related to defects in ciliary function.
Interactions between stem cells and their microenvironment in the human limbal niche
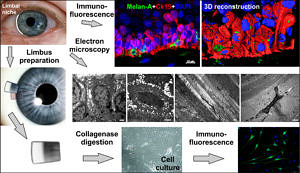 Another focus of my research are stem cells in the cornea. The corneal epithelium acts as a protective barrier on the anterior ocular surface and is essential for corneal transparency and visual function. During both homeostasis and repair, the corneal epithelium is maintained by self-renewing stem/progenitor cells at a transition zone termed limbus, which separates the cornea from the surrounding conjunctiva. Depletion of this stem cell reservoir and/or destruction of its niche microenvironment can cause severe ocular surface disease and significant visual deterioration. Our goal is to analyze this niche microenvironment, regulating the stem cell activities, to replicate the biological niche in vitro, and to establish stem cell based therapies for ocular surface disorders.
Another focus of my research are stem cells in the cornea. The corneal epithelium acts as a protective barrier on the anterior ocular surface and is essential for corneal transparency and visual function. During both homeostasis and repair, the corneal epithelium is maintained by self-renewing stem/progenitor cells at a transition zone termed limbus, which separates the cornea from the surrounding conjunctiva. Depletion of this stem cell reservoir and/or destruction of its niche microenvironment can cause severe ocular surface disease and significant visual deterioration. Our goal is to analyze this niche microenvironment, regulating the stem cell activities, to replicate the biological niche in vitro, and to establish stem cell based therapies for ocular surface disorders.
Methods
In our experimental approach, we combine neuroanatomical, immunocytochemical, biochemical, molecular, cell biological and physiological methods to investigate the sensory tissues of human, wild type and genetically modified mice.
Support
Deutsche Forschungsgemeinschaft DFG (Einzelförderung GI 770)
Dr. Hertha und Helmut Schmauser-Stiftung
Universitätsbund Erlangen-Nürnberg e.V.
Collaborations
My projects are embedded in international collaborations as well as in scientific networks at the University of Erlangen-Nuremberg.
PD Dr. Christian Thiel, Institute of Human Genetics, University of Erlangen-Nuremberg, Germany
Prof. Ronald Roepman, Department of Human Genetics, Molecular Biology of Ciliopathies, Radboud University Nijmegen Medical Centre, Netherlands
Prof. J. Kremers, Department of Ophthalmology, University of Erlangen- Nuremberg, German
Prof. Dr. Felix B. Engel, Nephropathologische Abteilung, University of Erlangen-Nuremberg, Germany
Dr. Stefan Geimer, Electron Microscopy Laboratory, Institute for Cell Biology, University of Bayreuth, Germany
Prof. Dr. Marius Ueffing, Dr. Karsten Boldt, Institute for Ophthalmic Research, Molecular Biology of Retinal Degenerations, University of Tübingen, Germany
Prof. Dr. Ralf Enz, Institute for Biochemistry, University of Erlangen-Nuremberg, Germany
Prof. Dr. Uwe Wolfrum, Department of Cell and Matrix Biology, Johannes Gutenberg-University Mainz, Germany
Prof. Regina Trollmann, Pediatric Clinic, University of Erlangen-Nuremberg, Germany
Prof. Michel Cayouette, Vasanth Ramamurthy, Department of Biology, Cellular Neurobiology Research Unit, Institut de recherches cliniques de Montreal (IRCM), Montreal, Canada
Prof. Yoshitaka Ono, Prof. Mikiko Takahashi, Biosignal Research Center, Kobe University, Japan
Selected Publications
- Braunger BM, Gießl A, Schlötzer-Schrehardt U (2023) The Blood-ocular Barriers and their Dysfunction: Anatomy, Physiology, Pathology. Klin Monbl Augenheilkd. 2023 May;240(5):650-661. doi: 10.1055/a-2063-8957. Epub 2023 May 19. PMID: 37207638
- Baur R, Karl F, Böttcher-Loschinski R, Stoll A, Völkl S, Gießl A, Flamann C, Bruns H, Schlötzer-Schrehardt U, Böttcher M, Schewe DM, Fischer T, Jitschin R, Mackensen A, Mougiakakos D (2023) Accumulation of T-cell-suppressive PD-L1(high) extracellular vesicles is associated with GvHD and might impact GvL efficacy. J Immunother Cancer. 2023 Mar;11(3):e006362. doi: 10.1136/jitc-2022-006362. PMID: 36898735
- Böttcher M, Böttcher-Loschinski R, Kahlfuss S, Aigner M, Gießl A, Mackensen A, Schlötzer-Schrehardt U, Tüting T, Bruns H, Mougiakakos D (2022) CLL-Derived Extracellular Vesicles Impair T-Cell Activation and Foster T-Cell Exhaustion via Multiple Immunological Checkpoints. Cells. 2022 Jul 12;11(14):2176. doi: 10.3390/cells11142176. PMID: 35883619
- Rama R, Derlig K, Vießmann N, Gossmann R, Oriold F, Gießl A, Brandstätter JH, Enz R, Dahlhaus R (2022) Simiate and the focal adhesion kinase FAK1 cooperate in the regulation of dendritogenesis. Sci Rep. 2022 Jul 4;12(1):11274. doi: 10.1038/s41598-022-14460-y. PMID: 35787638
- Szewczykowski C, Mardin C, Lucio M, Wallukat G, Hoffmanns J, Schröder T, Raith F, Rogge L, Heltmann F, Moritz M, Beitlich L, Schottenhamml J, Herrmann M, Harrer T, Ganslmayer M, Kruse FE, Kräter M, Guck J, Lämmer R, Zenkel M, Gießl A, Hohberger B (2022) Long COVID: Association of Functional Autoantibodies against G-Protein-Coupled Receptors with an Impaired Retinal Microcirculation. Int J Mol Sci. 2022 Jun 29;23(13):7209. doi: 10.3390/ijms23137209. PMID: 35806214
- Wen J, Lyu P, Stolzer I, Xu J, Gießl A, Lin Z, Andreev D, Kachler K, Song R, Meng X, Cao S, Guggino G, Ciccia F, Günther C, Schett G, Bozec A (2022) Epithelial HIF2α expression induces intestinal barrier dysfunction and exacerbation of arthritis. Ann Rheum Dis. 2022 Jun 16:annrheumdis-2021-222035. doi: 10.1136/annrheumdis-2021-222035. Online ahead of print. PMID: 35710307
- Zenkel M, Hoja U, Gießl A, Berner D, Hohberger B, Weller JM, König L, Hübner L, Ostermann TA, Gusek-Schneider GC, Kruse FE, Pasutto F, Schlötzer-Schrehardt U (2022) Dysregulated Retinoic Acid Signaling in the Pathogenesis of Pseudoexfoliation Syndrome. Int J Mol Sci. 2022 May 26;23(11):5977. doi: 10.3390/ijms23115977. PMID: 35682657
- Zhou X, Trinh-Minh T, Tran-Manh C, Gießl A, Bergmann C, Györfi AH, Schett G, Distler JHW (2022) Impaired Mitochondrial Transcription Factor A Expression Promotes Mitochondrial Damage to Drive Fibroblast Activation and Fibrosis in Systemic Sclerosis. Arthritis Rheumatol. 2022 May;74(5):871-881. doi: 10.1002/art.42033. Epub 2022 Mar 29. PMID: 34807516
- Li S, Zenkel M, Kruse FE, Gießl A, Schlötzer-Schrehardt U (2022) Identification, Isolation, and Characterization of Melanocyte Precursor Cells in the Human Limbal Stroma. Int J Mol Sci. 2022 Mar 29;23(7):3756. doi: 10.3390/ijms23073756. PMID: 35409129
- Hohberger B, Harrer T, Mardin C, Kruse F, Hoffmanns J, Rogge L, Heltmann F, Moritz M, Szewczykowski C, Schottenhamml J, Kräter M, Bergua A, Zenkel M, Gießl A, Schlötzer-Schrehardt U, Lämmer R, Herrmann M, Haberland A, Göttel P, Müller J, Wallukat G (2021) Case Report: Neutralization of Autoantibodies Targeting G-Protein-Coupled Receptors Improves Capillary Impairment and Fatigue Symptoms After COVID-19 Infection. Front Med (Lausanne). 2021 Nov 18;8:754667. doi: 10.3389/fmed.2021.754667. eCollection 2021. PMID: 34869451
- Polisetti N, Gießl A, Zenkel M, Heger L, Dudziak D, Naschberger E, Stich L, Steinkasserer A, Kruse FE, Schlötzer-Schrehardt U (2021) Melanocytes as emerging key players in niche regulation of limbal epithelial stem cells. Ocul Surf. 2021 Oct;22:172-189. doi: 10.1016/j.jtos.2021.08.006. Epub 2021 Aug 20. PMID: 34425298
- Lux UT, Ehrenberg J, Joachimsthaler A, Atorf J, Pircher B, Reim K, Kremers J, Gießl A, Brandstätter JH (2021) Cell Types and Synapses Expressing the SNARE Complex Regulating Proteins Complexin 1 and Complexin 2 in Mammalian Retina. Int J Mol Sci. 2021 Jul 29;22(15):8131. doi: 10.3390/ijms22158131. PMID: 34360929
- Hohberger B, Ganslmayer M, Lucio M, Kruse F, Hoffmanns J, Moritz M, Rogge L, Heltmann F, Szewczykowski C, Fürst J, Raftis M, Bergua A, Zenkel M, Gießl A, Schlötzer-Schrehardt U, Lehmann P, Strauß R, Mardin C, Herrmann M (2021) Retinal Microcirculation as a Correlate of a Systemic Capillary Impairment After Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Front Med (Lausanne). 2021 Jul 9;8:676554. doi: 10.3389/fmed.2021.676554. eCollection 2021. PMID: 34307408
- Schlötzer-Schrehardt U, Latta L, Gießl A, Zenkel M, Fries FN, Käsmann-Kellner B, Kruse FE, Seitz B (2021) Dysfunction of the limbal epithelial stem cell niche in aniridia-associated keratopathy. Ocul Surf. 2021 Jul;21:160-173. doi: 10.1016/j.jtos.2021.06.002. Epub 2021 Jun 6. PMID: 34102310
- Schlötzer-Schrehardt U, Zenkel M, Strunz M, Gießl A, Schondorf H, da Silva H, Schmidt GA, Greiner MA, Okumura N, Koizumi N, Kinoshita S, Tourtas T, Kruse FE (2021) Potential Functional Restoration of Corneal Endothelial Cells in Fuchs Endothelial Corneal Dystrophy by ROCK Inhibitor (Ripasudil). Am J Ophthalmol. 2021 Apr;224:185-199. doi: 10.1016/j.ajo.2020.12.006. Epub 2020 Dec 11. PMID: 33316261
- Andreev D, Liu M, Kachler K, Llerins Perez M, Kirchner P, Kölle J, Gießl A, Rauber S, Song R, Aust O, Grüneboom A, Kleyer A, Cañete JD, Ekici A, Ramming A, Finotto S, Schett G, Bozec A (2021) Regulatory eosinophils induce the resolution of experimental arthritis and appear in remission state of human rheumatoid arthritis. Ann Rheum Dis. 2021 Apr;80(4):451-468. doi: 10.1136/annrheumdis-2020-218902. Epub 2020 Nov 4. PMID: 33148700
- Genetics of Exfoliation Syndrome Partnership; Li Z, Wang Z, Lee MC, Zenkel M, Peh E, Ozaki M, Topouzis F, Nakano S, Chan A, Chen S, Williams SEI, Orr A, Nakano M, Kobakhidze N, Zarnowski T, Popa-Cherecheanu A, Mizoguchi T, Manabe SI, Hayashi K, Kazama S, Inoue K, Mori Y, Miyata K, Sugiyama K, Higashide T, Chihara E, Ideta R, Ishiko S, Yoshida A, Tokumo K, Kiuchi Y, Ohashi T, Sakurai T, Sugimoto T, Chuman H, Aihara M, Inatani M, Mori K, Ikeda Y, Ueno M, Gaston D, Rafuse P, Shuba L, Saunders J, Nicolela M, Chichua G, Tabagari S, Founti P, Sim KS, Meah WY, Soo HM, Chen XY, Chatzikyriakidou A, Keskini C, Pappas T, Anastasopoulos E, Lambropoulos A, Panagiotou ES, Mikropoulos DG, Kosior-Jarecka E, Cheong A, Li Y, Lukasik U, Nongpiur ME, Husain R, Perera SA, Álvarez L, García M, González-Iglesias H, Fernández-Vega Cueto A, Fernández-Vega Cueto L, Martinón-Torres F, Salas A, Oguz Ç, Tamcelik N, Atalay E, Batu B, Irkec M, Aktas D, Kasim B, Astakhov YS, Astakhov SY, Akopov EL, Giessl A, Mardin C, Hellerbrand C, Cooke Bailey JN, Igo RP Jr, Haines JL, Edward DP, Heegaard S, Davila S, Tan P, Kang JH, Pasquale LR, Kruse FE, Reis A, Carmichael TR, Hauser M, Ramsay M, Mossböck G, Yildirim N, Tashiro K, Konstas AGP, Coca-Prados M, Foo JN, Kinoshita S, Sotozono C, Kubota T, Dubina M, Ritch R, Wiggs JL, Pasutto F, Schlötzer-Schrehardt U, Ho YS, Aung T, Tam WL, Khor CC (2021) Association of Rare CYP39A1 Variants With Exfoliation Syndrome Involving the Anterior Chamber of the Eye. JAMA. 2021 Feb 23;325(8):753-764. doi: 10.1001/jama.2021.0507. PMID: 33620406
- Polisetti N, Gießl A, Li S, Sorokin L, Kruse FE, Schlötzer-Schrehardt U (2020) Laminin-511-E8 promotes efficient in vitro expansion of human limbal melanocytes. Sci Rep. 2020 Jul 6;10(1):11074. doi: 10.1038/s41598-020-68120-0. PMID: 32632213
- Falk N, Joachimsthaler A, Kessler K, Lux UT, Noegel AA, Kremers J, Brandstätter JH, Gießl A (2019) Lack of a Retinal Phenotype in a Syne-2/Nesprin-2 Knockout Mouse Model. Cells. 2019 Oct 11;8(10):1238. doi: 10.3390/cells8101238. PMID: 31614616
- Taylor RW, Mahmoodabadi RG, Rauschenberger V, Giessl A, Schambony A, Sandoghdar V (2019) Interferometric scattering microscopy reveals microsecond nanoscopic protein motion on a live cell membrane. Nature Photonics. 2019 Apr 15; doi: 1038/s41566-019-0414-6
- Falk N, Kessler K, Schramm SF, Boldt K, Becirovic E, Michalakis S, Regus-Leidig H, Noegel AA, Ueffing M, Thiel CT, Roepman R, Brandstätter JH, Gießl A (2018) ) Functional analyses of Pericentrin and Syne-2 interaction in ciliogenesis. J Cell Sci. 2018 Aug 17;131(16):jcs218487. doi: 10.1242/jcs.218487. PMID: 30054381
- Mühlhans J, Gießl A (2012) Pericentrin in health and disease: Exploring the patchwork of Pericentrin splice variants. Commun Integr Biol. 2012 Jul 1;5(4):304-7. doi: 10.4161/cib.20363. PMID: 23060948
- Mühlhans J, Brandstätter JH, Gießl A (2011) The Centrosomal Protein Pericentrin Identified at the Basal Body Complex of the Connecting Cilium in Mouse Photoreceptors. PLoS One. 2011;6(10):e26496. doi: 10.1371/journal.pone.0026496. Epub 2011 Oct 21. PMID: 22031837
- Thiel C, Kessler K, Gießl A, Dimmler A, Shalev SA, von der Haar S, Zenker M, Zahnleiter D, Stöss H, Beinder E, Abou Jamra R, Ekici AB, Schröder-Kress N, Aigner T, Kirchner T, Reis A, Brandstätter JH, Rauch A (2011) NEK1 mutations cause Short-Rib Polydactyly Syndrome Type Majewski. Am J Hum Genet. 2011 Jan 7;88(1):106-14. doi: 10.1016/j.ajhg.2010.12.004. PMID: 21211617